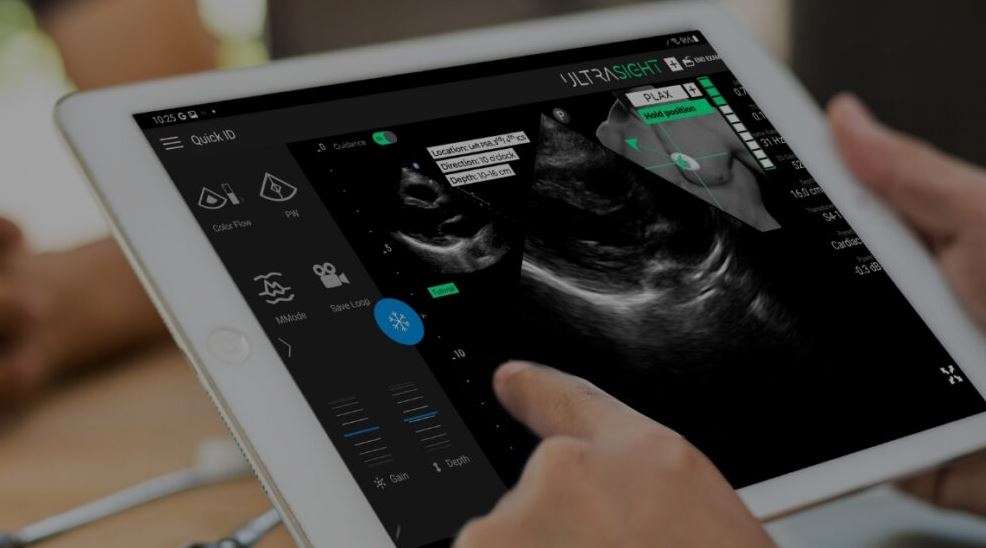
AI cardiac imaging startup, UltraSight, has been granted FDA clearance for its AI-based cardiac ultrasound technology, promising increased accessibility of cardiac imaging for patients by enabling healthcare professionals with no prior sonography experience to capture ultrasound images.
Heart disease accounts for more than 8 million annual emergency department admissions in the US, according to the CDC, and 30 million patients in the US are living with heart disease, requiring regular cardiac monitoring.
The startup's AI-powered technology, using deep geometric machine learning, offers healthcare professionals a more efficient way to expedite patient triage and treatment, thereby enhancing clinical confidence. Furthermore, it could boost access to care for chronic heart disease patients by introducing cardiac ultrasound into local communities, potentially improving patient compliance to essential treatments.
UltraSight's AI Guidance software has been approved for use in two-dimensional transthoracic echocardiography (2D-TTE) for adult patients, specifically for capturing the 10 standard views of the heart. The FDA clearance follows a pivotal study demonstrating that, with real-time guidance of the ultrasound probe and feedback on image quality, healthcare professionals without ultrasound experience can capture diagnostic-quality images.
The software, designed as an accessory for point of care ultrasound systems, is compatible with the Philips Lumify Ultrasound System. It works by predicting the position of the ultrasound probe relative to the heart, based on the ultrasound video stream, thereby guiding the user to acquire diagnostic-quality cardiac images.
UltraSight (previously On Sight) has raised $15 million from investors including Atain Specialty Insurance Company, Yozma Group Asia, and the Weizmann Institute of Science (StartupHub.ai data). The company currently employs 29 people.