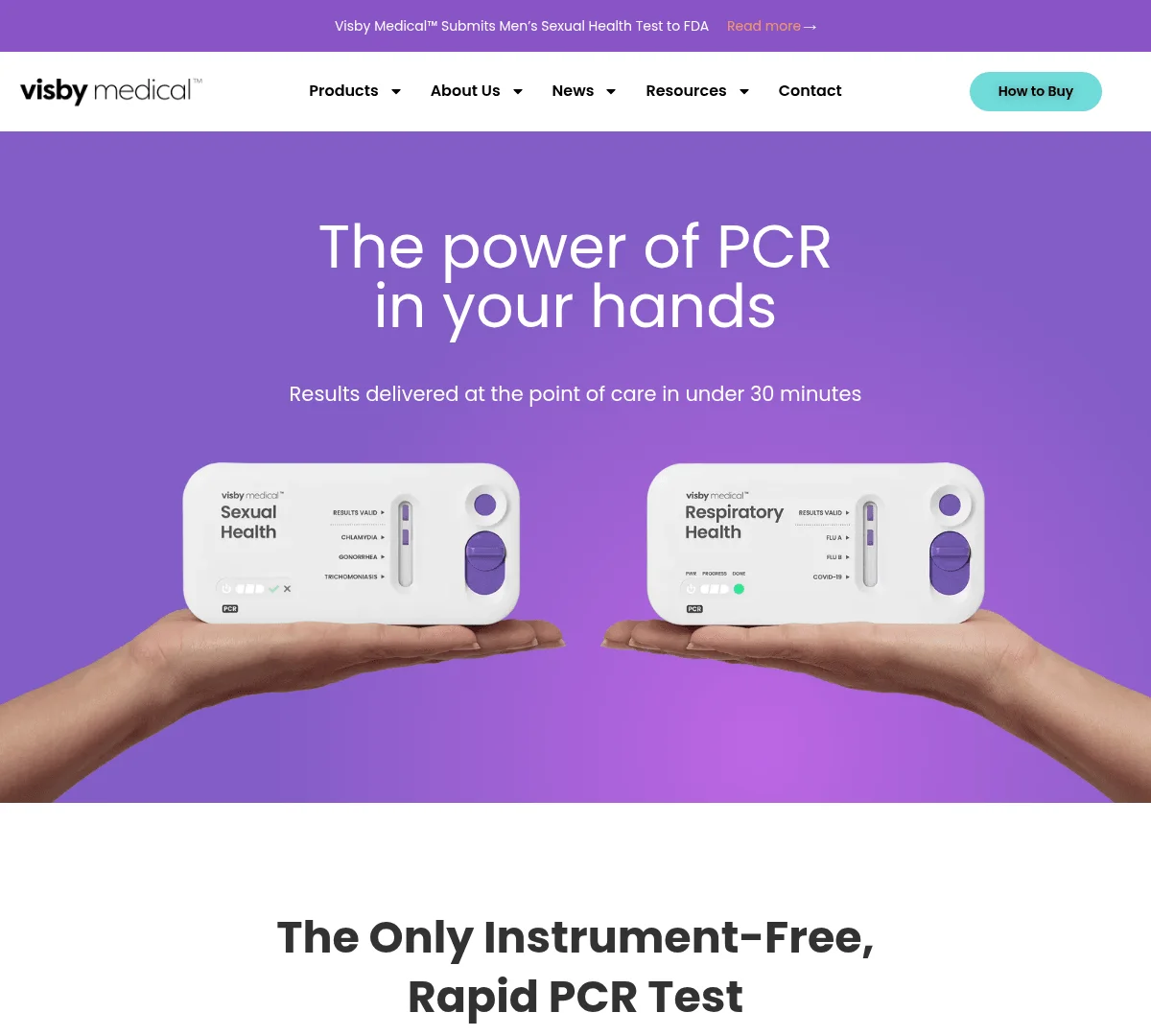
Visby Medical has raised up to $65 million in a round led by Catalio Capital Management to launch the first FDA-authorized at-home PCR test for sexually transmitted infections (STIs). The company’s Women’s Sexual Health Test detects and differentiates three major infections—Chlamydia, Gonorrhea, and Trichomoniasis—with lab-grade accuracy in under 30 minutes, using a single-use, instrument-free PCR device.
Unlike traditional diagnostics that require lab equipment and processing delays, Visby Medical’s technology integrates automated real-time PCR (RT-PCR) into a compact, disposable format operable via a smartphone app. Positive results trigger an automatic referral to a telemedicine provider, streamlining diagnosis, consultation, and treatment.
The company’s core diagnostic platform is protected by a broad portfolio of U.S. and international patents, covering its handheld, self-contained PCR device and packaging. Visby holds over 80 patent filings globally, with coverage across key markets including the U.S., China, and Australia.
This funding round includes participation from ND Capital, Cedars Sinai Medical Center, Blue Water Life Science Advisors, Pitango Ventures, and John Doerr. Catalio’s Isaac Ro joins Visby’s board as an observer, and Chuck Alpuche, COO at Imperative Care and former executive at Insulet and PepsiCo, joins as an independent director to support manufacturing scale-up and cost reduction.
In addition to the STI test, Visby offers a Respiratory Health Test that detects Flu A, Flu B, and SARS-CoV-2 (COVID-19) using the same instrument-free PCR platform, though this version remains available only in point-of-care settings.
Founded in 2012 and headquartered in San Jose, California, Visby Medical is positioning itself as a leader in ultra-rapid molecular diagnostics, aiming to deliver the accuracy of lab results anywhere, anytime.